Palbociclib In The Treatment Of Breast Cancer
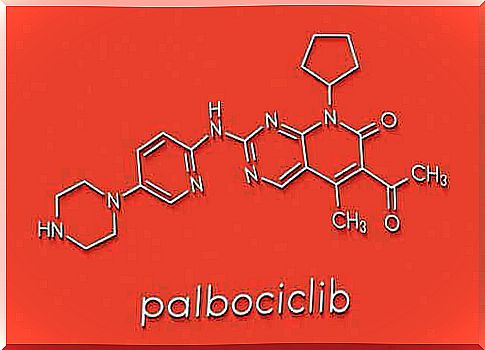
Palbociclib, marketed under the name Ibrance by the pharmaceutical company Pfizer, is a new “targeted therapy” against breast cancer. It is the first selective inhibitor of a class of enzymes called cyclin-dependent kinases 4 and 6 (CDK 4/6).
It is currently given in combination with hormone therapy (such as letrozole). It is intended for postmenopausal women with HR estrogen receptor positive and HER-2 negative metastatic breast cancer.
Currently indicated for this group of patients, its efficacy and safety is being evaluated – always in combination with hormone therapy – on tumors in other stages of development (eg the initial phase) or as adjuvant therapy.
Approval of palbociclib
The approval of this drug followed a different path than usual. It was in fact accelerated, in the light of data emerging from a phase 2 study called PALOMA-1.
Given to women with metastatic breast cancer and who had not received any systemic treatment, it showed promising results. It was then approved without waiting for the results of phase 3. Palbociclib doubled progression-free survival compared to administration of letrozole alone.
Rapid approval of the drug was also supported by positive results from another study called PALOMA-2 in phase 3 . This study involved 666 women with RH-positive and HER-2 negative metastatic breast cancer.
Randomized women were divided into two groups: palbociclib + letrozole therapy and placebo with letrozole. Compared to placebo, palbociclib improved median progression-free survival by nearly 10 months. 15 months of the placebo group versus almost 25 months of the palbociclib group.
In addition, more than half of the patients who received this drug had a significant reduction in tumor size after treatment.
However, time is not sufficient to determine whether this therapy improves overall survival in this type of patient. Therefore, the study is not yet completed and the monitoring of patients continues.
How does palbociclib work?
As mentioned, palbociclib is an inhibitor of cyclin-dependent kinase enzymes 4 and 6 (CDK 4 and CDK 6). This type of cyclins play an important role in the signaling pathways that lead to cell proliferation.
CDK 4/6 inactivates the retinoblastoma protein through phosphorylation. Once the protein is inactivated, cells can proliferate.
Conversely, if cyclins are inhibited and the retinoblastoma is active, i.e. dephosphorylated, transcription factors are inhibited with the result that the cells do not multiply.
Undesirable reactions of palbociclib
The drug is taken orally with an initial dose of 125 mg. However, depending on the adverse reactions developed by the patient, the dose can be reduced to 100 mg or even 75 mg if necessary.
Permanent discontinuation of treatment following an adverse reaction was required in 8% of patients treated with palbociclib and letrozole.
The most frequent side effects are:
- Neutropenia : decrease in neutrophils, a type of white blood cell. Normally, if it is grade 3 it usually disappears by reducing the dose of the drug.
- Fatigue.
- Anemia.
- Leukopenia : decrease in leukocytes.
- Infections.
- Diarrhea.
- Loss of appetite.
Conclusion
The marketing of palbociclib represented an important step forward in the treatment of metastatic breast cancer. At the same time, other CDK 4/6 inhibitors, such as ribociclib are offering similar results in clinical trials.
New indications for this drug are currently being studied. For example in early stage cancer or in the treatment of the first isolated locoregional recurrence of breast cancer.
It should not be forgotten that prevention is one of the most important factors in the fight against breast cancer, as are periodic check-ups.